Click here to download the PDF report, or the single page abstract.
By Joel Johnson
Lab-grown organs have been widely proclaimed as the way of the future, largely due to media hype. However, although there have been many advances into the culture of tissue and replacement organs in the last few years, the only functional organs that have been successfully grown are simple organs such as skin and ears. The other options – using mechanical organs or organs from other species – also have their own issues. Currently the only viable source of replacement organs is from a human donor.
Although there are a few records of organ transplants, such as skin and cornea, in the late 19th and early 20th century, the modern era of organ transplants did not begin until around 1950, when the first successful kidney transplant was performed (1). Artificial organs did not make it onto the stage until almost 20 years later, when an artificial heart was implanted for almost three days, in 1969 (2). However research into artificial and lab-grown organs is occurring at a faster pace than ever before, and it is possible that human donations may eventually become a thing of the past.
Currently, there is a chronic shortage of donor organs. In Australia alone, there was an average of 1,640 people on the waiting list at any time in 2015, yet only 1,239 organ transplants occurred for the whole year (3). Worldwide, the problem is even greater. Although there were almost 120,000 organ transplants in 2014 (4), it is estimated that 2 million people currently need an organ transplant (5).
Cell Culture
Culturing cells is an essential preliminary step to developing replacement organs. The field of cell culture has grown rapidly since the 1880s, when Sydney Ringer developed a particular mixture of salts which allowed him to maintain the beating heart of a tortoise for several hours (6, 7). Nowadays, nearly all the cells in the human body are able to be cultured in the laboratory.
Artificial skin, used in extreme burn cases, has been around since the 1970s (8). However, early ‘skin’ only served as a framework for new skin cells to grown on, and no hair follicles or sweat glands ever formed. Only in April this year were researchers able to create a complex, living skin that included structures such as hairs and glands (9). They did this by turning cells obtained from mouse gums into stem cells, and then implanting them into the skin of other mice.
Not only did the implanted tissue contain sebaceous glands and hair follicles, but it also made connections with the surrounding muscle and nervous tissue. This indicates that the skin will function as real skin would. Although clinical trials are still years off, this development brings the prospect of growing fully functioning skin one step closer.
Cell culture is also an integral part of a new method of manufacturing viral vaccines. A live virus is added to cultured cells grown from modified kidney tumour cells (10). Once the virus has multiplied sufficiently, it is separated from the cells and chemically inactivated. It is then split into pieces and the surface proteins are extracted and used in the vaccine. Its main advantage over the traditional method – growing the virus in chicken eggs – is that the vaccine supply can be increased by simply adding more culture cells. The cell-culture method has been used to make influenza vaccines (11).
Another vaccine production method – the recombinant DNA method – also involves cell cultures. The genes encoding for the hemagglutinin (HA) surface proteins of the virus are inserted into another virus which only infects army worms (a type of moth). The modified virus is then introduced to a culture of army worm cells, where it reprograms them to produce the HA proteins that the pathogenic virus carries. The HA proteins are then separated, purified and used in the viral vaccine. This method takes as little as 6 weeks to develop (compared to 4½ to 8 months for the cell-culture method), and vaccine production can also be easily ramped up (10).
Recombinant DNA cell cultures, where bacteria or other cells are reprogrammed to produce a specific compound, can also be used to produce enzymes, synthetic hormones and antibodies (6). Cell cultures are also used for studying how diseases affect the body.
Replacement Organs
Mechanical replacements organs have been used very little throughout history, due to the challenge of replicating the extreme complexity of organs using only biocompatible materials. The only mechanical organ that has enjoyed limited success is the artificial heart; an artificial kidney has been created, but not tested in humans (12). It is generally accepted that the main goal of mechanical organs is to keep the patient alive until a replacement donor organ becomes available (12).
Other non-living structures that have been created are simple passive scaffolds, such as the first artificial skin, and an artificial windpipe. The artificial windpipe was hailed as “the first of many off-the-shelf organs” (13), but unfortunately six of the eight patients that received it have died. The surgeon responsible is being investigated for alleged ethical breaches (14).
An artificial ear
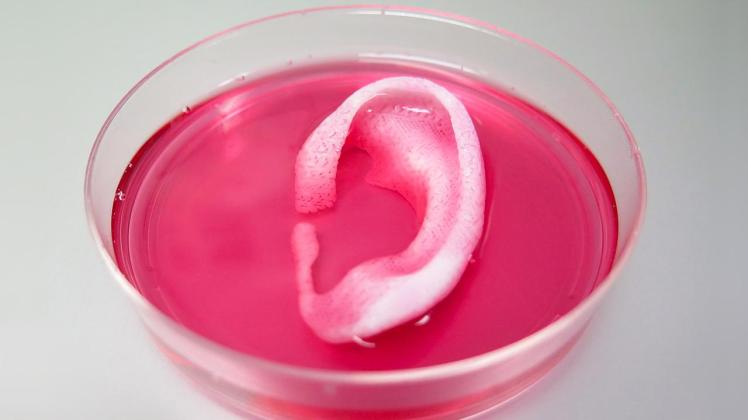
http://www.sciencemag.org/news/2016/02/tissue-printer-creates-lifelike-human-ear
One possibility for reducing the organ crisis is using xenografts – transplanting organs from one species into another. Valves from pig hearts have been used to replace malfunctioning heart valves in humans for over 40 years (15); however there is still a risk of transplant rejection. Sterilisation procedures and immunosuppressive drugs allow pig valves to be used; however whole organ transplants have not – until recently – been considered possible.
In 2015, Harvard Medical School researchers using the recently developed CRISPR Cas9 technique to edit over 60 genes in pig embryos, removing a number of viruses embedded in the pig genome (16). Without these viruses, and with further gene editing, it may be possible to use pig organs in human recipients (17).
Improvements in immunosuppressive drugs will also play a role in allowing organ transplants between different species. A major problem with commonly used immunosuppressive drugs such as cyclosporine is that infections can be life threatening, as the immune system cannot respond as it normally would (18). However in April this year, National Institute of Health researchers reported that they were able to keep pig hearts alive in baboons for over two years, thanks to new antibodies which prevent graft rejection without disabling the immune system (19). This is a remarkable step up from early xenotransplants, where the survival time could be measured in minutes (19).
Xenotransplants can pose moral problems, as they do not consist of human tissue. It may be possible to dispose of living donors entirely and grow the organs. This approach sounds very futuristic, but way back in 1997 researchers grew a structure in the shape of a human ear, on the back of a mouse (20). Somewhat surprisingly, there was no genetic engineering involved at all. The ear was grown by seeding cow cartilage cells onto a biodegradable ear-shaped scaffold, then implanting the structure into a mouse, which merely provided nutrients for the cow cells to grow. To prevent the mouse rejecting the cow cells, a variety that had no immune system – thanks to a natural mutation – was used.
One of the most promising options is 3D printed organs, which have begun to make their debut (17). As early as 2003, a patent existed for printing a matrix of living cells (21); however it is only recently that technology sophisticated enough to create whole organs has existed. In February, researchers reported that they were able to print living cells onto a stiff, hydrogel structure (22). They also left tiny holes in the structure to allow oxygen to reach the interior cells. Thanks to these two improvements, they were able to print life-size tissue structures such as a nose and an outer ear. Incredibly, the structures developed blood vessels when implanted into rats, suggesting that 3D printed tissues will be accepted by human recipients, and function like normal organs.
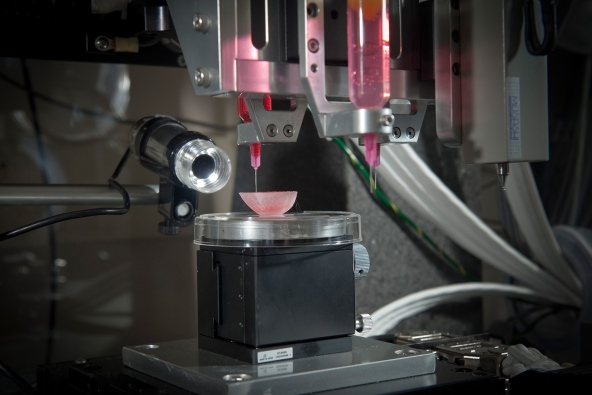
http://www.nature.com/news/the-printed-organs-coming-to-a-body-near-you-1.17320
Simple, flat structures like skin and veins have been successfully grown and transplanted into humans; however solid organs like liver and kidneys are still too complex for printing (17). Indeed, some researchers doubt that it will ever be possible to print complex structures like kidneys (23).
A similar approach is to encourage groups of cells to grow into organs, thus allowing them to create their own structure. Stem cells, which have the potential to become any cell in the body, have been used to create noses, ears, windpipes, heart tissue, blood vessels and even a simple liver (17). The process is comparable to that of 3D printing, with the stem cells placed in a nutrient rich gel, and left to grow. Like 3D printing, more complex organs typically do not function correctly, and it is thought that growing functioning organs suitable for transplantation will take many years and cost tens of millions of dollars (17).
The field of replacement organs has advanced significantly in the last 20 years, and development appears to be occurring faster than ever. For example, a search for replacement organs on Science Direct shows 5,822 papers for the year 2015, 56% more than the 3,741 papers in 2005 (24). However, virtually all replacement organs are still in the experimental phase; only a handful have been trialled in humans.
Despite this, it is highly likely that simpler organs grown in the laboratory, such as skin and veins, will be used as alternatives to human organs. It is more dubious whether complex structures like kidneys and liver will ever be able to be grown. Even optimistic predictions indicate that it will take at least ten years to develop them. In the meantime, human donors will continue to be the main source of replacement organs, as they have for the last 60 years.
References
- Little Company of Mary Hospital and Health Care Centers 2010, First successful organ transplant, Little company of Mary, 1950. Available from: http://lcmhealthnews.org/first-successful-organ-transplant-little-company-of-mary-1950/. [23 August 2016].
- Madrigal, A 2010, The world’s first artificial heart, The Atlantic. Available from: http://www.theatlantic.com/technology/archive/2010/10/the-worlds-first-artificial-heart/63949/. [8 August 2016].
- Organ and Tissue Authority 2016, Organ donation and transplantation outcomes, Government of Australia. Available from: http://www.donatelife.gov.au/sites/default/files/OTA%20Factsheet%20-%20Organ%20Outcomes.pdf. [8 August 2016].
- Global Observatory on Donation and Transplantation 2016, Global Observatory on Donation and Transplantation. Available from: http://www.transplant-observatory.org/. [8 August 2016].
- Sami, M 2015, Organ donation shortage: NSW hospital uses recycled and diseased kidneys for dialysis patients, ABC News. Available from: http://www.abc.net.au/news/2015-09-11/nsw-hospital-uses-recycled-and-diseased-kidneys/6768224. [8 August 2016].
- Wikipedia 2016, Cell culture. Available from: https://en.wikipedia.org/wiki/Cell_culture. [11 August 2016].
- Ringer, S & Buxton, DW 1887, ‘Upon the similarity and dissimilarity of the behaviour of cardiac and skeletal muscle when brought into relation with solutions containing sodium, calcium and potassium salts’, The Journal of Physiology, vol. 8, no. 5, pp. 288-295. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1485095/. [11 August 2016].
- Medical Discoveries 2016, Artificial skin. Available from: http://www.discoveriesinmedicine.com/Apg-Ban/Artificial-Skin.html. [13 August 2016].
- Science Daily 2016, Growing skin in the lab. Available from: https://www.sciencedaily.com/releases/2016/04/160401144507.htm. [13 August 2016].
- Berkowitz, B, Maloney, B & Stanton, L 2009, Three ways to make a vaccine, Washington Post. Available from: http://www.washingtonpost.com/wp-dyn/content/graphic/2009/11/24/GR2009112401834.html?sid=ST2009112401941. [22 August 2016].
- Centers for Disease Control and Prevention 2016, Cell-based flu vaccines. Available from: http://www.cdc.gov/flu/protect/vaccine/cell-based.htm. [22 August 2016].
- Sifferlin, A 2013, Artificial and implanted organs, Time magazine. Available from: http://healthland.time.com/2013/06/06/5-discoveries-that-will-change-the-future-of-organ-transplants/slide/artificial-organs/. [13 August 2016].
- Coghlan, A 2011, Man receives world’s first synthetic windpipe, New Scientist. Available from: https://www.newscientist.com/article/dn20671-man-receives-worlds-first-synthetic-windpipe/. [22 August 2016].
- Cyranoski, D 2016, Artificial-windpipe pioneer under scrutiny again, Nature. Available from: http://www.nature.com/news/artificial-windpipe-pioneer-under-scrutiny-again-1.19272. [13 August 2016].
- Benway, R, Benedict, L & Boldt, M n.d., History. Available from: http://www.pages.drexel.edu/~rjb56/history.htm. [8 August 2016].
- Reardon, S 2015, Gene-editing record smashed in pigs, Nature. Available from: http://www.nature.com/news/gene-editing-record-smashed-in-pigs-1.18525. [8 August 2016].
- Grenoble, R 2015, These advances in lab-grown organs might save your life one day, The Huffington Post. Available from: http://www.huffingtonpost.com.au/entry/lab-grown-organs-transplant-technology_us_562122cee4b08d94253ee660. [8 August 2016].
- Spenceley, M, Weller, B, Mason, M, Fullerton, K, Tsilemanis, C, Evans, B, Ladiges, P, McKenzie, J & Batterham, P 2004, Heinemann Queensland science project: biology – a contextual approach, Pearson Australia, Melbourne.
- Servick, K 2016, Researchers keep pig hearts alive in baboons for more than 2 years, Science online. Available from: http://www.sciencemag.org/news/2016/04/researchers-keep-pig-hearts-alive-baboons-more-2-years. [9 August 2016].
- Kruszelnicki, K 2006, Mouse with a human ear, ABC. Available from: http://www.abc.net.au/science/articles/2006/06/02/1644154.htm. [11 August 2016].
- Boland, T, Wilson, WC & Xu, T 2003, Ink-jet printing of viable cells, US Patent 7051654.
- Rogers, N 2016, Tissue printer creates lifelike human ear, Science online. Available from: http://www.sciencemag.org/news/2016/02/tissue-printer-creates-lifelike-human-ear. [9 August 2016].
- Ledford, H 2015, The printed organs coming to a body near you, Nature. Available from: http://www.nature.com/news/the-printed-organs-coming-to-a-body-near-you-1.17320. [9 August 2016].
- Science Direct 2016, Replacement organs. Available from: http://www.sciencedirect.com/science?_ob=ArticleListURL&_method=list&_ArticleListID=-1035174852&_sort=r&_st=13&view=c&md5=bcf2952e152a70faaf30a92cdf27d161&searchtype=a. [11 August 2016].